- About-Ethics Committee
- Genesis of Ethics Committee
- Organogram of Ethics Committee
- Standard Operating Procedure
- IEC Review Procedures
- Trial Status
- IEC Meeting Schedules
- IEC Annexure For Principal Investigators
- Accreditation / Registrations
- IEC Fee Notification
- Clinical Trial Audit / Inspection
- Events
- Thrust Areas
- Contacts
- FAQs
The Vice Chancellor of KLE Academy of Higher Education and Research, Belagavi establish the Institutional Ethics Committee. The Ethics committee offers essential assistance, resources, and autonomy to ensure the smooth operation and effective decision-making during the protocol review process. All research that involves human participants must be carried out in accordance with three fundamental ethical principles, encompassing the following:
Respect for persons
Beneficence
Justice
ESTD YEAR : 2009
| SL.No | SOPs | SOP Code | Page |
| I | Preparation of SOPs for Ethics committee for clinical trials | SOP/01/V-8.5 | 1-13 |
| II | Constituting the Ethics Committee for Research on Human Subjects
|
SOP/02/ V-8.5 SOP/02/ V-8.5 SOP/02/ V-8.5 SOP/02/ V-8.5 |
14-25 26-37 38-41 42-47 |
| III | Initial Review Procedures
|
SOP/06/ V-8.5 SOP/07/ V-8.5 SOP/08/ V-8.5 SOP/09/ V-8.5 SOP/10/ V-8.5 SOP/11/ V-8.5 |
48-66 67-73 74-90 91-97 98-102 103-108 |
| IV | Protocol Amendments, Continuing Review and End of Study | ||
| V | Monitoring and Evaluation of Adverse Events | ||
| VI | Monitoring Protocol Implementation/Compliances | ||
| VII | Study Monitoring Visit by IEC Members/Secretariat | ||
| VIII | Preparation and Review Meeting Agenda and Minutes of meeting | ||
| IX | Managing Study Files | ||
| X | Evaluating an IEC of KAHER | ||
| XI | Subjects/Patients recruitment strategies | ||
| XII | Review of Biomedical and Health Research and CDSCO-Clinical trials During COVID-19 Pandemic |
Management of protocol submissions
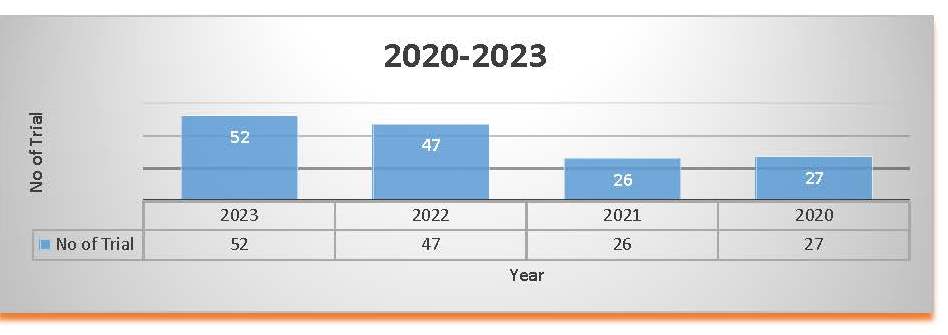
Trial Status Table 2020 to 2023
Through CTRI website or www.klehospital.org
Within 21 days [After full board meeting-EC clearance will get within 07 working days]
INR-75000 per protocol review.
The Registrar, KAHER, Belagavi
Yes, ECR/211/Inst/2013/RR-19 Under ND&CT Rules, 2019
Yes, EC/NEW/INST/2021/1726
Yes, FWA: 00024127
- > IORG#-0001102
- – IRB00001499 JNMC Ethics Committee on Human Subjects Rsc IRB#1
- – IRB00012930 KLE Academy of Higher Education and Research IRB#2