Institutional Ethics Committee
Of
KLE Academy of Higher Education and Research
JNMC Campus, KLEs Dr.Prabhakar Kore Hospital and MRC, Nehru Nagar, Belagavi-Karnataka-590010
INDEX
The Vice Chancellor of KLE Academy of Higher Education and Research, Belagavi establish the Institutional Ethics Committee. The Ethics committee offers essential assistance, resources, and autonomy to ensure the smooth operation and effective decision-making during the protocol review process. All research that involves human participants must be carried out in accordance with three fundamental ethical principles, encompassing the following:
Respect for persons
Beneficence
Justice
Toggle title
Toggle content goes here, click edit button to change this text.
 |
Index of Standard Operating Procedures of Ethics Committee VERSION-8.5 |
| Sl.No | SOPs | SOP code | Page No. |
| I. Preparation of SOPs for Ethics committee for clinical studies | |||
|
01 |
Writing, Reviewing, Distributing and Amending Standard Operating Procedures for institutional Ethics Committees | SOP/01/V-8.5 | 1-13 |
| II. Constituting the Ethics Committee for Research on Human Subjects | |||
| 02 | Constitution of an IEC | SOP/02/ V-8.5 | 14-25 |
| 03 | Confidentiality/Conflict of Interest Agreement | SOP/03/ V-8.5 | 26-37 |
| 04 | Training Personnel and Ethics Committee Members | SOP/04/ V-8.5 | 38-41 |
| 05 | Selection and Responsibilities of Independent consultants | SOP/05/ V-8.5 | 42-47 |
| III. Initial Review Procedures | |||
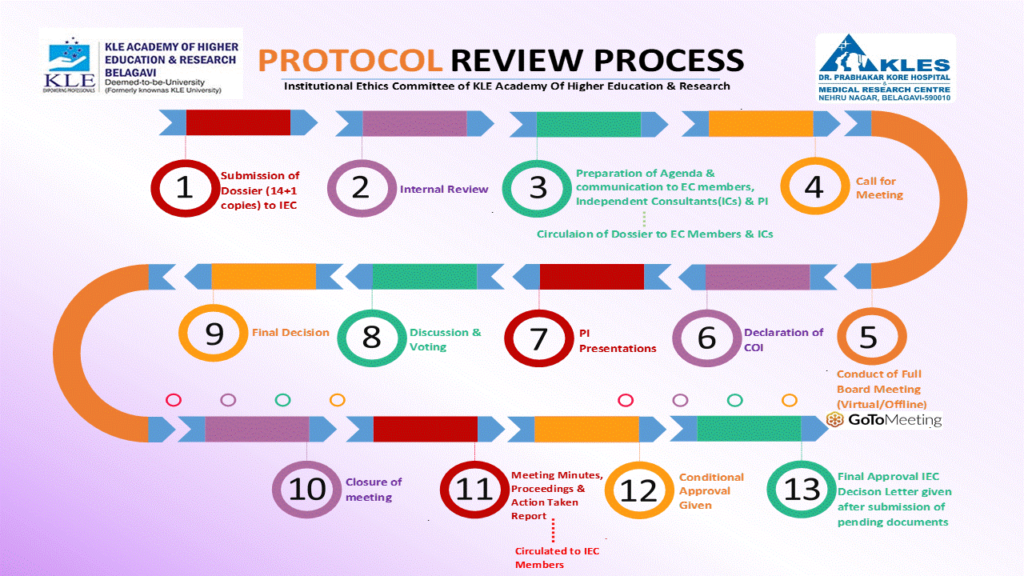
| 06 | Management of protocol submissions | SOP/06/ V-8.5 | 48-66 |
| 07 | Expedited Review | SOP/07/ V-8.5 | 67-73 |
| 08 | Initial Review of submitted protocol | SOP/08/ V-8.5 | 74-90 |
| 09 | Vulnerable populations | SOP/09/ V-8.5 | 91-97 |
| 10 | Audio Visual (AV) recording of the informed consent process | SOP/10/ V-8.5 | 98-102 |
| 11 | Review of Resubmitted protocols | SOP/11/ V-8.5 | 103-108 |
| IV. Protocol Amendments, Continuing Review and End of Study | |||
| 12 | Review of Protocol Amendments | SOP/12/ V-8.5 | 109-115 |
| 13 | Continuing Review of Study Protocol | SOP/13/ V-8.5 | 116-121 |
| 14 | Review of Final report | SOP/14/ V-8.5 | 122-125 |
| V. Monitoring and Evaluation of Adverse Events | |||
| 15 | Review of Serious Adverse Events (SAE) Reports | SOP/15/ V-8.5 | 126-136 |
| VI. Monitoring Protocol Implementation | |||
| 16 | Intervention in Protocol Deviation/Non-Compliance/ Violation | SOP/16/ V-8.5 | 137-143 |
| 17 | Response to Complaints, Queries & Requests | SOP/17/ V-8.5 | 144-152 |
| 18 | Management of Study Termination | SOP/18/ V-8.5 | 153-157 |
| VII. Site Monitoring Visits | |||
| 19 | Site Monitoring visit | SOP/19/ V-8.5 | 158-165 |
| VIII. Preparation of Review Meeting Agenda and Communication Records | |||
| 20 | Agenda Preparation, Meeting Procedures and Minutes | SOP/20/ V-8.5 | 166-186 |
| IX. Managing Study Files | |||
| 21 | Maintenance of active study files | SOP/21/ V-8.5 | 187-191 |
| 22 | Archival and retrieval of documents | SOP/22/ V-8.5 | 192-198 |
| 23 | Maintaining Confidentiality of IEC Documents | SOP/23/ V-8.5 | 199-205 |
| X. Evaluating an IEC KAHER | |||
| 24 | Audit and Inspection | SOP/24/ V-8.5 | 206-212 |
| XI. Subjects/Patients recruitment strategies | |||
| 25 | Subjects/Patients recruitment strategies | SOP/25/ V-8.5 | 213-220 |
| 26 | Continuous improvement: a corrective and preventive action (CAPA | SOP/26/ V-8.5 | 221-228 |
| XII. Review of Biomedical and Health Research and CDSCO-Clinical trials During COVID-19 Pandemic | SOP/27/ V-8.5 | 229-230 | |
IEC OF KLE ACADEMY OF HIGHER EDUCATION AND RESEARCH
MEETING SCHUDELES [Tentative List-2024]
| Sl.No | Month | Meeting Day and Date | Time |
| 1 | January | Friday – 19 | 3.30 PM |
| 2 | February | Thursday – 15 | 3.30 PM |
| 3 | March | Thursday – 14 | 3.30 PM |
| 4 | March | Thursday – 28 | 3.30 PM |
| 5 | April | Tuesday – 16 | 3.30 PM |
| 6 | May | Friday – 17 | 3.30 PM |
| 7 | June | Monday – 10 | 3.30 PM |
| 8 | July | Wednesday – 27 | 3.30 PM |
| 9 | July | Tuesday – 16 | 3.30 PM |
| 10 | August | Monday – 12 | 3.30 PM |
| 11 | September | Wednesday – 12 | 3.30 PM |
| 12 | September | Wednesday – 25 | 3.30 PM |
| 13 | October | Monday -21 | 3.30 PM |
| 14 | November | Monday – 11 | 3.30 PM |
| 15 | November | Thursday – 28 | 3.30 PM |
| 16 | December | Friday – 21 | 3.30 PM |
Note :
- Above mentioned date and time are tentative
- If the proposed dates are changed due to technical reasons, the fresh dates will be communicated.
- For details of the schedule of meeting you can contact the secretariat
- In some cases, IEC will schedule the Two meetings in a month*
Member-Secretary of IEC
K.L.E. Academy of Higher Education & Research, Belagavi
LIST OF FORMS
| Sl. No. | Documents | Forms Download |
| 1 | Confidentiality Agreement Form for IEC members | FORM No:06 |
| 2 | Conflict of Interest Agreement Form for IEC members | FORM No:07 |
| 3 | Confidentiality Agreement Form for Guest Attendees to IEC- KLE Meetings | FORM No:08 |
| 4 | Confidentiality Agreement Form for Independent consultants | FORM No:09 |
| 5 | Confidentiality Agreement for Non-members Requesting Copy of IEC Documents | FORM No:10 |
| 6 | Study Assessment Form -INDEPENDENT CONSULTANT | FORMS:14 PART-A |
| 7 | Checklist for Investigator | Form No: 15 |
| 8 | Study Assessment Form for New protocol | Form No: 16 |
| 9 | Clinical Trial Agreement Checklist | Form No: 17 |
| 10 | Study Principal Investigator CV Format | Form No: 18 |
| 11 | Contents of the proposed protocol for the Conducting Clinical Trial | Form No: 19 |
| 12 | Guidance for Protocol Submission | Form No:22 |
| 13 | Format for Summary and Detailed Protocol | Form No:23 |
| 14 | Undertaking by investigators | Form No:24 |
| 15 | Guide to Placebo Justification | Form No:25 |
| 16 | IEC approval letter format | Form No:26 |
| 17 | Assent Form-Template | Form No:28 |
| 18 | Submission of amended study protocol template | Form No: 29 |
| 19 | Amended study protocol assessment form | Form No:30 |
| 20 | Annual Report Template | Form No:31 |
| 21 | Study Completion report template | Form No:32 |
| 22 | Data elements for reporting serious adverse events occurring in a clinical trial or bioavailability or bioequivalence study | Form No: 33 |
| 23 | SAE Reporting Template | Form No:34 |
| 24 | Deviation/Non-Compliance/Violation Record | Form No:35 |
| 25 | Clinical Research Stakeholder’s Request/Complaint Form | Form No:36 |
| 26 | study termination template | Form No:41 |
| 27 | Checklist for IEC members Monitoring Visit | Form No:42 |
| 28 | Format of an Agenda | Form No:43 |
| 29 | Format for IEC Minutes meetings | Form No:44 |
| 30 | IEC Documents Retrieval Record Form | Form No:46 |
| 31 | Audit and Inspection Checklist | Form No:50 |
| 32 | Confidentiality Agreement Form for Auditors/inspectors | Form No:51 |
| ACCREDIATATIONS | |
| NABH : Accreditation for Ethics Committee Clinical Trial | |
| FERCAP : Forum for Ethical Review Committees in the Asian and Western Pacific | |
| RECOGNITIONS | |
| CDSCO : The Central Drugs Standard Control Organization | |
| DHR Registration : Department of Health Research | |
| OHRP : Office for human Research protections | |
- IEC Secretariat
Mobile No: 0831-2470400
Mail ID: kleclinicalresearch@gmail.com
- Member Secretary
- Mobile No: +91-9902687176
- Mail ID: geetanjalisalimath@gmail.com
- Chairperson
Mobile No: +91-9449033133
- Mail ID: drsubarnaroy@gmail.com
Clinical Trial Participants Feedback Link : https://forms.gle/HEC4CTepS68292gU8
| Questions | Answer |
| How can obtain the contact information of the site investigator for KLE? | Through CTRI website or www.klehospital.org |
| What is the timelines for the submission of Dossier to IEC of KAHER? | Within 21 days [After full board meeting-EC clearance will get within 07 working days] |
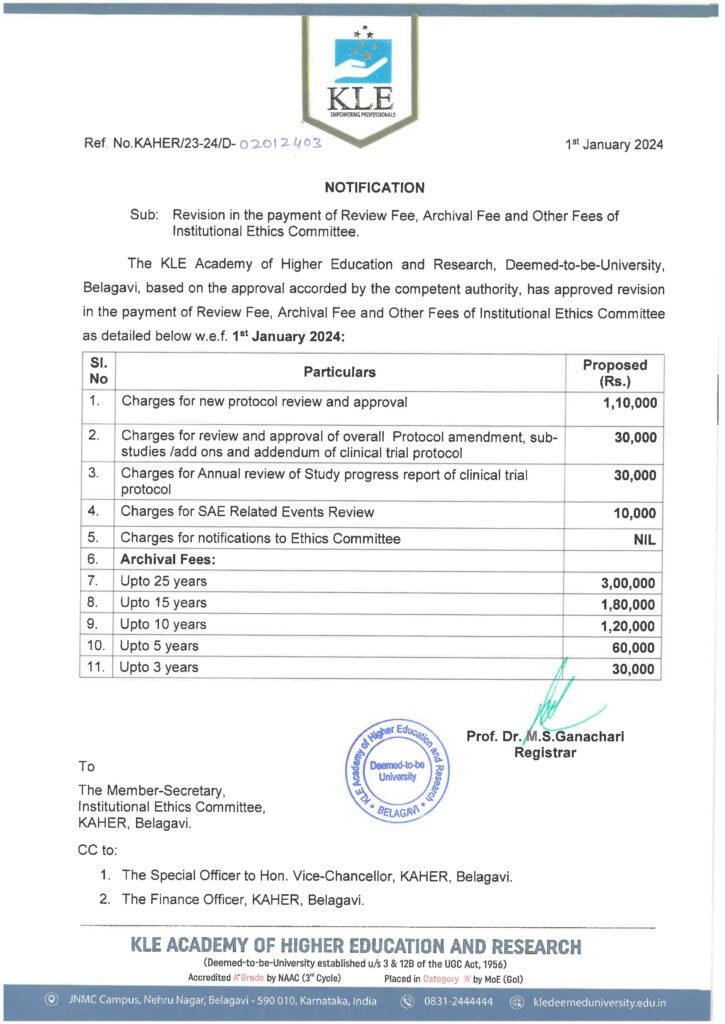
| What is the Ethics committee fee? | INR-110000 per protocol review-Refer-IEC Notification |
| In whose name should the cheque be drawn for payment of EC fees? | The Registrar, KAHER, Belagavi |
| Does the EC have DCGI approval? | Yes, ECR/211/Inst/2013/RR-19 Under ND&CT Rules, 2019 |
| Does the EC have DHR approval? | Yes, EC/NEW/INST/2021/1726 |
| Does the site is registered OHRP-FWA? | Yes
|
| Does the site registered DUNS? | YES |